At present, the determination of total nitrogen in domestic garbage generally uses the CJ/T103-1999 determination method of total nitrogen in municipal solid wastes, which is a semi-micro JK method. However, due to the drawbacks of the method, such as cumbersome operations and time-consuming operations, it is necessary to search for a rapid and accurate method. The method for the determination of total nitrogen in domestic wastes is provided, and reference is provided for the compilation and revision of relevant standards such as the “General Test Method for Chemical Characteristics of Municipal Solid Wasteâ€. The authors conducted a relevant experimental study on the determination of total nitrogen in domestic waste by the method of determining nitrogen.
Food Processor 8 In 1
Food Processor 8 In 1 including juicer, blender, fliter, chopper, grinder and citrus.
Multi-functional vegetable fruit plastic bowl food processor is now a must-have item in your daily life.
This machine comes with a large capacity blending jar and chopping bowl. S/S sharp blades, different function tools..
Nowadays, a multi-functional food precessor is necessary at home. You can prepare jam, nuts, flour, seasoner, sauce... and you also can use it as meat chopper, vegetable fruit blender, food mixer etc...
Description for Food Processor 8 In 1
8820 copper & aluminum Motor
220V, 50HZ, 450-1000W,
VDE 2 PIN plug 2/3 speeds
Stainless steel processing blades
Food Processor 8 In 1,Manual Baby Food Processor 8 in 1,Multifunctional 8 in 1 food processor,Blender 8 in 1 Food Processor Flying Electronic Co., Ltd , https://www.flyingelectronic.com
1 Instruments and reagents: Automatic nitrogen determination instrument (top), digestion furnace, automatic sample determination tube (large test tube) and commonly used laboratory glassware.
Reagents: sulfuric acid (analytically pure), 40% sodium hydroxide (analytically pure), catalyst (potassium sulfate: copper sulfate: selenium powder = 100:10:1, analytically pure), 0.1% methyl red ethanol solution (analysis Pure), 0. 1% bromocresol green ethanol solution (analytic purity), 1% boric acid (analytically pure, 1 L boric acid solution were respectively added 0.1% methyl red ethanol solution 7 mL and 0.1% bromine A Phenol green ethanol solution 10 mL, and adjust the pH to 4.5 5~5 5), anhydrous sodium carbonate (excellent grade pure), ethylenediaminetetraacetic acid disodium (reference reagent). The water used in the experiment was heavy distilled water.
0. 1 mol/L HCl standard solution configuration: Pipette 8. 5 mL of concentrated hydrochloric acid (analytical purity) in appropriate amount of water and make up to 1 000 mL, shake well, and then calibrate with anhydrous sodium carbonate. Accurately weigh dried anhydrous sodium carbonate 0,020 0 g in a conical flask, add 40 mL of freshly boiled and cooled water and 2 drops of methyl red-bromocresol green indicator (2 parts methyl red And 3 parts of bromocresol green mixed solution), titrated with hydrochloric acid until the solution changes from green to lavender, record the volume of the hydrochloric acid standard solution, calculate the concentration of hydrochloric acid standard solution according to the following formula, the calibration result needs to be taken with duplicate samples The average, while doing blank experiments.
C0=2×1000 m/106 (V-V0)×100%.
Where: c0 is the concentration of hydrochloric acid standard solution, mol/L; m is the mass of anhydrous sodium carbonate, g; V is the volume of hydrochloric acid standard solution consumed by the sample, mL; V0 is the blank sample consumed by hydrochloric acid The volume of the standard solution, mL; 2 is the number of moles of hydrochloric acid required to neutralize 1 mol of anhydrous sodium carbonate; 106 is the molar mass of anhydrous sodium carbonate, g/mol.
The concentration of the hydrochloric acid standard solution used in this experiment was calibrated to 0.117 1 mol/L.
2 Determination 2.1 Determination Principle After the sample is added to the catalyst and digested with concentrated sulfuric acid, various nitrogen-containing organic compounds are subjected to a complex pyrolysis reaction and converted to ammonia nitrogen. The alkalized and distilled ammonia is absorbed by boric acid. After the titration with the acid standard solution, the total nitrogen content of the domestic waste was calculated.
2. 2 Preparation of samples Samples of domestic waste collection, preparation and preservation of the implementation of CJ/T313-2009 domestic garbage sampling and analysis methods related provisions.
2. 3 Assay Procedure 2.3.1 Digestion of sample Weigh approximately 0.3 g of sample (accurate to 0·000 1 g) into the bottom of a large, dry tube, add 2 g of catalyst, and moisten with a small amount of water. Samples, add 8 mL of concentrated sulfuric acid, shake, cover a small neck funnel, set the digestion furnace heating at low temperature, when the reaction within the bottle is mild (about 1 h), adjust the temperature appropriately to keep the solution boiling. It is advisable to condense and return the acid mist in the upper 1/3 of the test tube. After 2 hours, the temperature is increased again. After the digestion solution turns to grayish green, the heating and cooling are stopped.
2.3.2 Distillation and Titration of Ammonia Prepare by distillation and titration according to the instructions of the automatic nitrogen determination instrument. Distill the digestion solution completely and titrate with hydrochloric acid standard solution.
2.3.3 blank experiment The blank experiment is performed in synchronization with the sample. The reagents and procedures used are the same as the sample, except that the sample is not added. Each batch of sample is subjected to 3 blank experiments. The blank experiment consumes the hydrochloric acid standard solution. The volume generally does not exceed 0.1 mL.
3 Measurement conditions 3.1 Digestion temperature Due to the large difference in physical composition of domestic garbage sample in different areas, the waste sample has complex components and inhomogeneity, which makes it difficult to digest the sample, and whether the sample digestion is complete or not directly affects the final determination. result. The digestion process and phenomena are shown in Table 1. 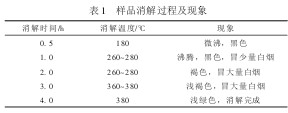
Table 1 Sample digestion process and phenomenon 3.2 Sulphuric acid addition The concentration of concentrated sulphuric acid in this experiment directly influences the degree of digestion and the digestion time of the sample. With the development of economic construction and improvement of people’s living standards, the physical composition of domestic waste has also changed along with it. The content of organic matter has increased significantly, and the content of inorganic matter has dropped significantly, which has brought certain difficulties to the digestion of samples. It is appropriate to add 8 mL of sulfuric acid.
4 QUALITY CONTROL AND GUARANTEE 4.1 Precision Two samples were measured in the laboratory, and 5 parallel samples were prepared for each sample. The results are shown in Table 2. 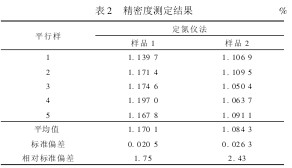
Table 2 Precision Measurement Result %
It can be seen from Table 2 that the relative standard deviations of the sample 1 and sample 2 measured by the automatic nitrogen determination method are 1.75% and 2.43%, respectively, which are in accordance with the general analysis method and the relative standard deviation (less than 5%). It is required that the determination of total nitrogen in household waste using the nitrogen determination method is appropriate and feasible.
4.2 Recovery rate of standard addition A certain amount of reference material is added to the sample to determine the recovery rate. This is a method commonly used by laboratories to determine the accuracy, and it is also a quality control method often used in the laboratory. The following points should be noted when carrying out the determination of the recovery rate of the spiked standard: (1) The morphology of the spiked substance should be similar to that of the analyte; (2) In any case, the amount of spiking should not exceed 3 times the amount of the analyte, and the amount of spiking should be as far as possible. It is equal or similar to the content of the sample in the sample, and pay attention to the influence on the sample volume; 3 The measured value after the addition of the standard should not exceed 90% of the upper limit of the method measurement.
Taking all these factors into consideration, this experiment used disodium EDTA (reference reagent) as a standard substance. Two samples were measured in the laboratory, and 5 samples were prepared for each sample. In the 0.3 g sample, 1.000 0% disodium EDTA was added, and 3 blanks were made at the same time. The standard recovery rate test, measured the average recovery rate of the two samples were 94. 03% and 94. 59%, blank average recovery rate of 91. 69%, see Table 3. 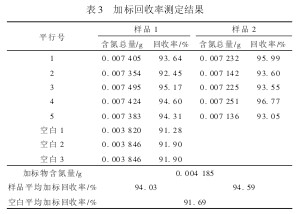
Table 3 Determination results of spiked recovery rate The qualification limits for the spiked recovery rate for environmental samples in early China were relatively strict, and the general requirement was 95% to 105%. However, due to the complexity of the environmental samples and the large difference in concentration, the determination of the environmental samples was prone to overshoot. Poor, so the judgment of the results may be inaccurate. In recent years, the requirements for the recovery rate of standard addition have been relaxed, and a control limit of 70% recovery rate has been proposed. In HJ/T 92-2002, the total discharge of water pollutants is monitored. The standard addition recovery rate proposed is 70% to 130% upper and lower extreme control limits. The determination results of this method are in accordance with the requirements of the chemical analysis for spike recovery. The parallel blank test consumes hydrochloric acid standard solution volume/mL Blank test assay result/mg indicates that the determination of total nitrogen in domestic waste is completely feasible with the nitrogen determination method. of.
4.3 Limits of Detection and Lower Limits of Measurement According to the whole process of sample analysis, n (n≥7) blank tests are repeated and the results are converted into the contents of the samples. The standard deviation of n times of parallel determination is calculated, according to the formula MDL. = St (n-1, 0. 99) Calculation method detection limit. Among them, MDL is the detection limit of the method, n is the number of parallel determinations of the sample, t(n-1, 0.99) is the degree of freedom n-1, the t-distribution when the confidence is 99% (single side), and S is n The standard deviation of sub-parallel determinations. In this experiment, the t-value of 11 blank experiments was measured continuously for 2.764, and the detection limit of the 4-fold method was usually used as the lower limit of measurement. The detection limit of this method was 0. 063 3 mg, and the lower limit of determination was 0. 253 2 mg, see Table 4. 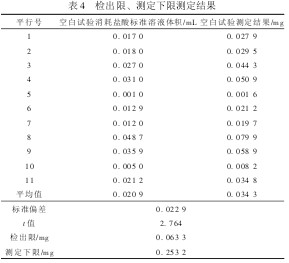
Table 4 Limits of detection and lower limit of measurement 5 Conclusion Scientific and effective disposal of domestic garbage is currently one of the issues of common concern. Through the analysis of the content of nutrients (total nitrogen) in domestic garbage, a treatment plan can be formulated for domestic garbage. In particular, to provide reference for the development of the classification and treatment of domestic waste. The automatic nitrogen determination method is accurate and convenient, with a low detection limit and a low detection limit, and has a good application value in the determination of total nitrogen in domestic garbage.

