Car Generator,Auto Generator,Generator For Auto,Generator For Automobile Chengdu Huachuan Electric Parts Co., Ltd. , https://www.chcd-global.com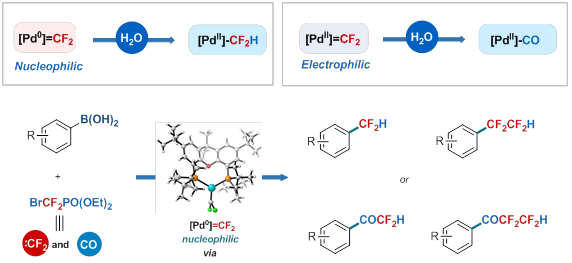
Shanghai Institute of Organic Materials and Other Progress in Metal Difluorocarbene Chemistry Research
[ Instrument Network Instrument Development ] Zhang Xingang, Key Laboratory of Organic Fluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, and Kendall N. Houk, a group of the University of California, Los Angeles, first completed the nucleophilic and electrophilic reaction of difluorocarbene by palladium metal. Sexual regulation and regulation can be used in organic synthesis, and achieve the "driving" of difluorocarbene. The nucleophilic and electrophilic palladium difluorocarbene ([Pd]=CF2) coexist in the same reaction system by valence state regulation of palladium metal, and can control the catalytic cycle to selectively generate different reaction products. This work was recently published in "Nature Chemistry" (DOI: 10.1038/s41557-019-0331-9). Zhang Xingang and Kendall N. Houk are co-authors. Dr. Xia Ping, Ph.D. student of Shanghai Institute of Organic Medicine, and Xue Xiaosong, a visiting scholar of Houk Group, are the co-first authors. Among them, Houk and Xue Xiaosong are responsible for the theoretical calculation part. Shanghai Organic Institute is the first communication unit. .
Difluorocarbene is an important chemical intermediate. Its preparation route is mature and it is widely used in the synthesis of fluorine-containing materials such as Teflon. From the perspective of organic synthesis route design, if difluorocarbene has a reaction diversity similar to that of traditional non-fluorocarbene intermediates, it will be an important synthon, which is widely used in the efficient synthesis of products with special properties and structures. Fluorine medicines, pesticides and material molecules. However, the inherent high electrophilicity of difluorocarbene makes its reaction type quite limited, and its reactive reactivity makes difluorocarbene difficult to apply to controlled organic synthesis reactions. Therefore, how to "tamed" and "driving" the "wild horse" of difluorocarbene and its widespread application in organic synthesis through reactive regulation has always been a challenging problem in organic fluorine chemistry.
Theoretically, the complex metal complex with difluorocarbene will change the electron cloud distribution of difluorocarbene, thus providing possibility for its reactive regulation. However, it is known that the isolated transition metal difluorocarbene complexes exhibit reaction inertness, and the catalytic cycle reaction involving metal difluorocarbene has rarely been reported. The industry lacks understanding and understanding of relevant theories, so that the metal II has not been realized. Fluconazole is used in catalytic coupling reactions that have been widely used. Until 2016, Zhang Xingang's group used the palladium as a catalyst to discover the first catalytic coupling reaction (MeDIC) involving metal difluorocarbene (Org. Lett. 2016, 18, 44), and realized the first large-scale cheap fluorine chemical raw materials. - Highly efficient catalytic conversion of chlorodifluoromethane (Nature Chemistry 2017, 9, 918).
Based on the above research, the research team used the change of the transition metal valence state to realize the regulation of difluorocarbene reactivity for the first time, so that difluorocarbene has nucleophilic or electrophilic reactivity in different metal valence states, thus achieving different Catalytic controllable synthesis of fluorine compounds. Based on this concept, they used palladium as a catalyst to achieve selective fluoroalkylation of aryl boronic acid compounds with simple and readily available BrCF2PO(OEt)2 as a difluorocarbene precursor; fine regulation of reaction conditions Controllable synthesis of four fluorine-containing compounds can be achieved, including difluoromethyl, tetrafluoroethyl aromatic compounds, aryl difluoromethyl groups, and aryl tetrafluoroethyl ketone compounds. The series of metal difluorocarbene participates in the controlled catalytic coupling reaction, excellent functional group compatibility, selective post-fluorine modification of complex medicines and pesticide molecules, and simultaneous synthesis of different fluorine-containing compounds in one pot method. Provides an efficient and easy way. Binding studies involving elementary reactions, computational chemistry, and key metal reaction intermediates indicate that both nucleophilic and electrophilic [Pd]=CF2 species are present in the reaction system; they first separated the zero-valent palladium difluorocarbene The compound [Pd0]=CF2 and proved to be highly nucleophilic and can undergo protonation reaction with water molecules. This is the only metal difluorocarbene species found to be protonated with water. The divalent palladium difluorocarbene complex [PdII]=CF2 has electrophilicity and can be hydrolyzed with water to form CO. This series has laid a theoretical foundation for the catalytic coupling reaction involving palladium difluorocarbene. It provides a new perspective for understanding other metal difluorocarbene chemistry and opens up a new path for the regulation of difluorocarbene reactivity.
The research was funded by the National Natural Science Foundation of China and the Strategic Science and Technology Special Project (Class B) of the Chinese Academy of Sciences.