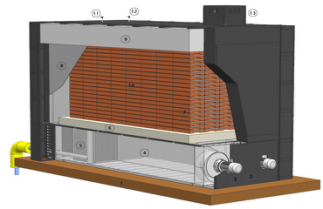
Solid Electric Heat Storage Boiler
100% consume abandon wind electric
Related Products:off-peak electric heat storage boiler,solid electric heat storage system,electric heat storage boiler,solid heat storage electric boiler,solid heat storage boiler,electric solid heat storage boiler.
Solid Electric Heat Storage Boiler Solid Electric Heat Storage Boiler,Electric Heat Storage Equipment,High Voltage Electric Heat Storage Boiler,Heat Energy Storage Electric Boiler Dalian Transen Technology Co.,Ltd. , http://www.transen-china.com

Specifications
Small volume
Lower operating costs
Save investment of transformer
Study on Electrochemical Separation of Copper-Moly
S. Chandler and DW Ersitingnao rich ore from the start and electrochemical characteristics of the study molybdenite Hui copper, copper seek separation using electrochemical characteristics - molybdenum minerals. Their research found that when no collector was added , the copper ore with a potential lower than -0.4V was in a reduced state:
Cu 2 S+H + +2e - → 2Cu+HS -
The copper is in an oxidized state at a potential of -0.5 ~ +0.2V:
Cu 2 S+H 2 O →[Cu 2+ , Cu 2 O, CuO, Cu(OH) 2 ]+[S, CuS, S 2 O 3 2- , etc.]+H + +e -
Obviously, the oxidation product is a poorly soluble and hydrophilic oxide or hydroxide. The molybdenum ore reacts in the oxidized state as:
MoS 2 +8OH - → MoO 4 2- +2[S]+4H 2 O+6e -
The oxidation product is a readily soluble MoO 4 2- .
When the addition of the collector diethyl dithio phosphate (DTP), the high potential (+ 0.2V), chalcocite and collector will generate ionic surface hydrophobic surface of the compound floats: (0.0V when DTP - ion discharge: DTP - → DTP 0 + e - )
Cu 2 S+(2-m)DTP 0 +mDTP - +2CuDTP+[S]+me -
Cu 2 S+(4-n)DTP 0 +nDTP - +Cu(DTP) 2 +[S]+ne -
The reverse reaction occurs at a low potential, and the collector on the surface of the chalcopyrite is desorbed without floating (the two reverse reaction potentials are -0.6 V and -0.9 v, respectively).
Molybdenum ore has little activity on the electrochemical oxidation of collectors. By using this difference, it can suppress molybdenum molybdenum at low potential and molybdenum float at high potential. The electrochemical flotation separation results of the copper samples containing half of the copper and molybdenum ore are shown in the table below.
Table Electrochemical flotation separation results (pre-adjusted potential -1.2V)
Flotation potential
Molybdenite recovery rate (%)
Copper recovery rate (%)
Copper ore in concentrate (%)
-1.2
-0.6
+0.3 (50mV/a)
+0.3 (5mV/a)
62
39
twenty one
17
14
6
63
100
18
14
75
86
[next]
GR Hyde pointed out that the collector adsorption on the surface of sulfide ore is an electrochemical process, and the floating of these minerals depends on the electrochemical potential or redox potential of the system. Cyanide and sulfide are used to lower the potential of the system so that certain minerals cannot adsorb the sulfhydryl-based collector. And potassium permanganate can improve the system potential.
Different minerals are activated at different potentials. Molybdenum ore is floatable at potentials below -0.5V, while sulphide ore and other copper minerals are more than -0.4V potential. (See Figures 1 and 2).
Figure 1 Relationship between electrochemical potential and flotation (according to the US Mine Laboratory Research Bureau)
Figure 2 Electrochemical potential and recovery of several copper sulfide minerals
Adding enough sulfide and cyanide to the slurry to control the system potential below -0.5V or -0.5V, it is possible to float molybdenum and inhibit copper to achieve copper-molybdenum separation.